Indicate Whether the Following Balanced Equations Involve Oxidation Reduction
Elementary differential equations and boundary. Indicate whether the following balanced equations involve oxidation-reduction.

Solved Problem 20 18 Part A Indicate Whether The Following Chegg Com
Were as to indicate whether the following balanced equations involve oxidation production.
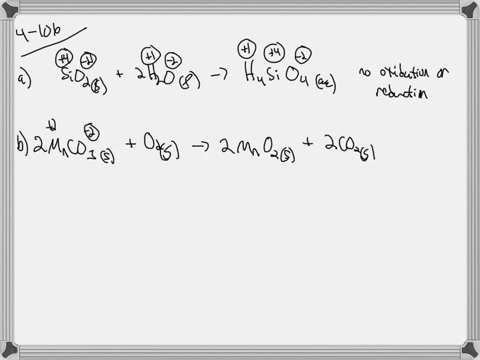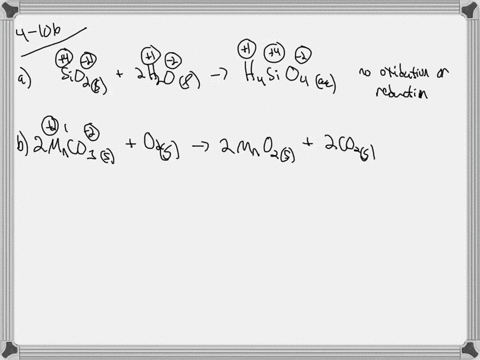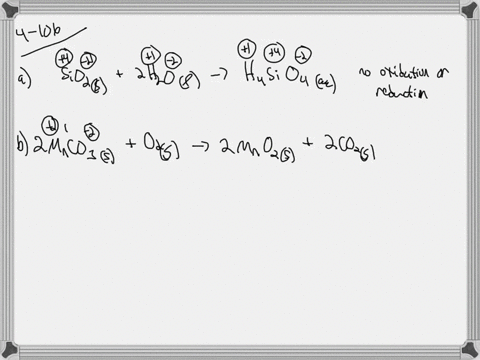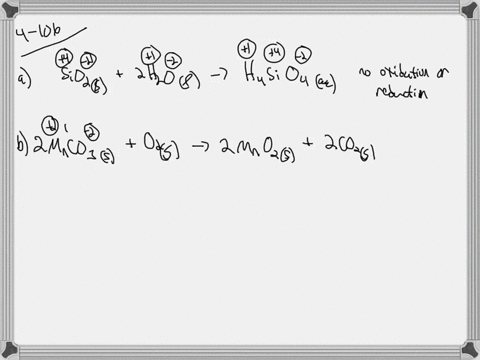
. If they do identify the elements that undergo changes in oxidation number. If they do identify the elements that undergo changes in oxidation number. Indicate whether the following balanced equations involve oxidation reduction.
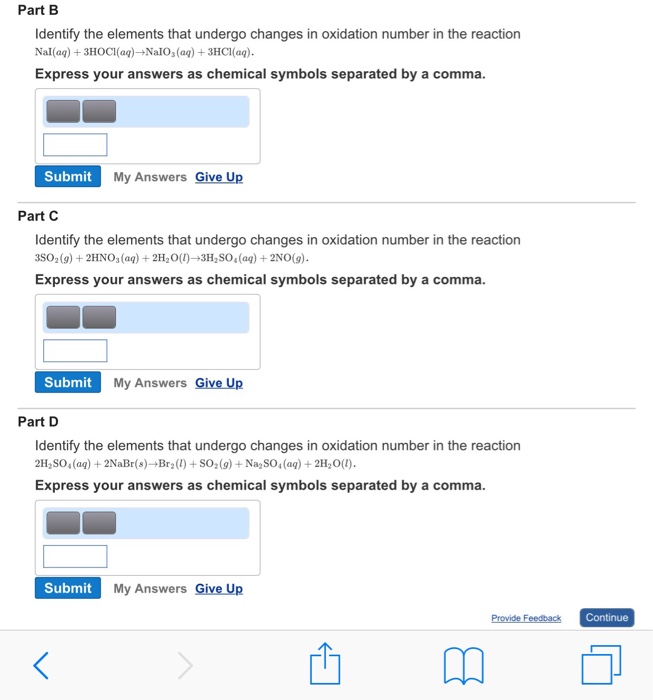
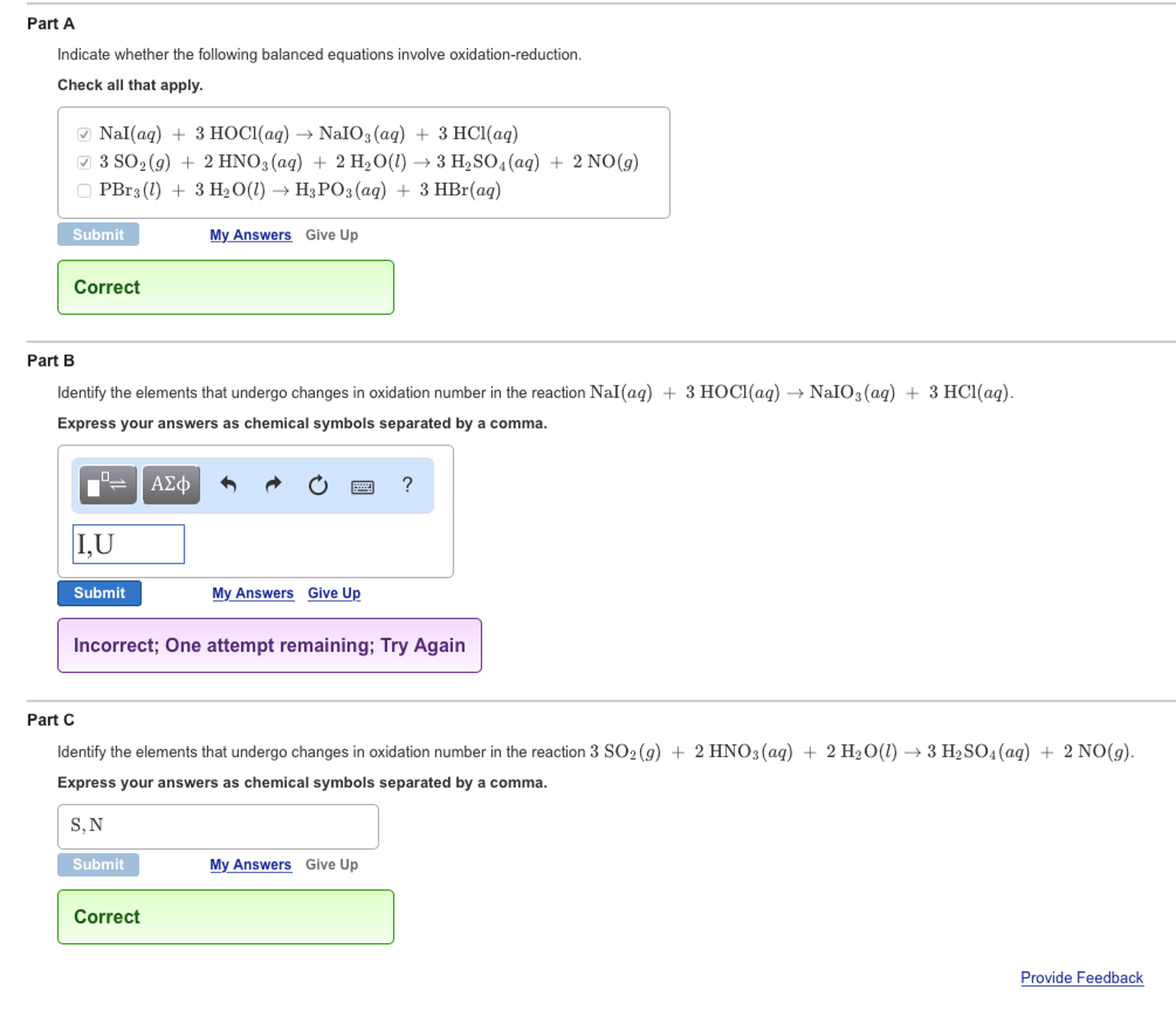
Check all that apply. Check all that apply. 2H2SO4aq 2NaBrsBr2l SO2g Na2SO4aq 2H2Ol 3SO2g 2HNO3aq 2H2Ol3H2SO4aq 2NOg NaIaq 3HOClaqNaIO3aq 3HClaq PBr3l 3H2OlH3PO3aq 3HBraq.
Indicate whether the following balanced equations involve oxidation-reduction. PBr_3l 3H_2OlRight Arrow H_3PO_3aq 3 HBraq Nalaq 3 HOClaq Right Arrow NalO_3aq 3 HClaq 3SO_2 g 2HNO_3aq 2H_2OlRight Arrow 3 H_2SO_4aq 2 NOg 2H_2SO_4aq. Indicate whether the following balanced equations involve oxidation-reduction.
APBr3l 3H2OlH3PO3aq 3HBraq bNaIaq 3HOClaqNaIO3aq HClaq c3SO2g 2HNO3aq 2H2Ol3H2SO4aq 2NOg. Indicate whether the following balanced equations involve oxidation-reduction. - 2AgNO3 aqCoCl2 aq2AgCl sCo NO32 aq - 2H2SO4 aq2NaBr sBr2 lSO2 gNa2SO4 aq2H2O l - 2PbO2 s2PbO sO2 g Question.
So first uh in this the first reaction which were given here is to silver nitrate. - 2AgNO3 aqCoCl2 aq2AgCl sCo NO32 aq - 2H2SO4 aq2NaBr sBr2. Indicate whether the following balanced equations involve oxidation-reduction.
In exercises 2426 use determinants to decide if the set of vectors is linearly independent. PBr3l 3H20l H3P03aq 3HBr aq No reciox b. A PBr3l 3 H2Ol H3PO3aq 3 HBraq b NaIaq 3 HOClaq NaIO3aq 3 HClaq.
A Ba2 aq 2OH- aq H2O2 aq 2ClO2 aq yields Ba ClO22 s 2H2O l O2 g b 2H2SO4 aq 2NaBr s yields Br2 l SO2 g Na2SO4 aq 2 H2O l Expert Answer. If they do identify the elements that undergo changes in oxidation number. If they do identify the elements that undergo changes in oxidation number.
A 2AgNO3 aq CoCl2aq ------. If they do identify the elements that undergo changes in oxidation number. Indicate whether the following balanced equations involve oxidation-reduction.
If they do identify the elements that undergo changes in oxidation number. If they do identify the elements that undergo changes in oxidation number. Check all that apply.
A utility company burns coal to generate electricity the cost c in dollars of removing p of the air. If they do identify the elements that undergo changes in oxidation numbera PBr31l2 3 H2O1l2 H3PO31aq2 3 HBr1aq2b NaI1aq2 3 HOCl1aq2 NaIO31aq2 3 HCl1aq2c 3 SO21g2 2 HNO31aq2 2 H2O1l2 3 H2SO41aq2 2 NO1g2. If they do identify the elements that undergo changes in oxidation number.
Nal aq 3HOCl aq Na O aq 3HCl aq CI reduced c. 2018 a PBr3 l 3H2O l H3PO3 aq 3HBr aq b NaI aq 3HOCl aq NaIO3 aq 3HCl aq c 3SO2 g 2HNO3 aq 2H2O l 3H2SO4 aq 2NO g. For each of.
Br2 l SO2g Na2SO4aq 2H2O l. Science Chemistry QA Library Indicate whether the following balanced equations involve oxidationreduction. 2PbO O2 g c 2H2SO4 aq 2NaBr s -------.
Indicate whether the following balanced equations involve oxidation-reduction. APBr3 l 3H2O lH3PO3 aq 3HBr aq bNaI aq 3HOCl aqNaIO3 aq HCl aq c3SO2 g 2HNO3 aq 2H2O l3H2SO4 aq 2NO g d 2H2SO4 aq 2NaBr sBr2 l Question. Indicate whether the following balanced equations involve oxidation-reductio 0810.
Solution for Indicate whether the balanced equation involves oxidationreduction. A 2 AgNO31aq2 CoCl21aq2 2 AgCl1s2 Co1NO3221aq2b 2 PbO21s2 2 PbO1s2 O21g2c 2 H2SO41aq2 2 NaBr1s2 Br21l2 SO21g2 Na2SO41aq2 2 H2O1l2. If they do identify the elements that undergo changes in oxidation numbers.
Indicate whether the following balanced equations involve oxidation-reduction. If they do identify the elements that undergo changes in oxidation number. 3S02 g 2HN03 aq 2H20 l 3H2S04aq 2NO g Il S YIU reduced.
Determine the truth value of each of these statements if the domain consists of all real numbers. Indicate whether the following balanced equations involve oxidation-reduction. Indicate whether the following balanced chemical equations involve oxidation-reduction redox.
If they do identify the elements that undergo changes in oxidation number. If they do ended find the elements that undergo changes in oxidation. Indicate whether the following balanced equations involve oxidation-reduction.
When the oxidized species is separated from the reduced species a balanced reaction can be written for each process oxidation or reduction that is called a half-reactionAll half-reactions must have electrons either as reactants for reduction. Indicate whether the following balanced equations involve oxidation-reduction. Up to 256 cash back Indicate whether the following balanced equations involve oxidation-reduction.
Indicate whether the following balanced equatins involve oxidation-reduction. If they do Identify the elements that undergo changes in oxidation number. So for part A just glancing at the change of oxygen from a peroxide um to Apollo atomic ion its not a peroxide.
2AgCl s CoNO32 aq b 2PbO2 s ------. For each of the following balanced oxidation-reduction reactions i identi 0526. If they do identify the elements that undergo changes in oxidation number.
Indicate whether the following balanced equations involve oxidation-reduction. Plus a couple chloride will produce two silver chlorides plus a cold nitrate. Check all that apply.
2AgNO 3 aq CoCl 2 aq 2AgCls CoNO 3 2 aq 2PbO 2 s 2 PbOs O 2 g 2H 2 SO 4 aq 2NaBrs Br 2 l SO 2 g Na 2 SO 4 aq 2 H 2 Ol One Class. Oxidation-reductions reactions always have an electron transfer from the oxidized species to the reduced species.
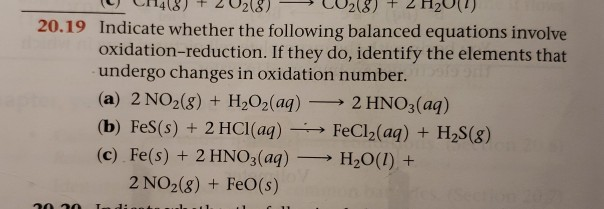
Solved 20 19 Indicate Whether The Following Balanced Chegg Com
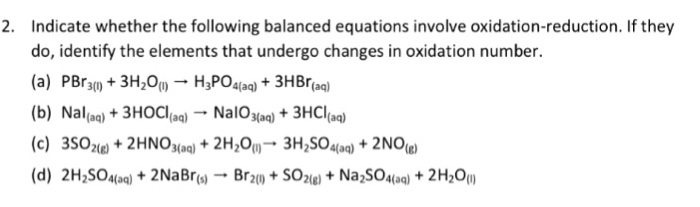
Solved Indicate Whether The Following Balanced Equations Chegg Com
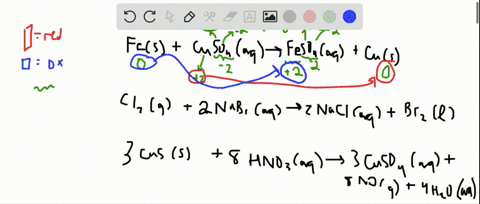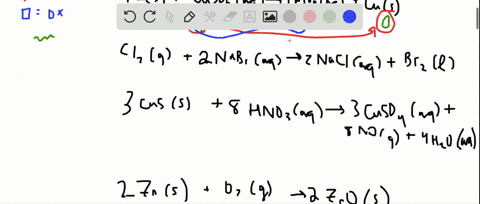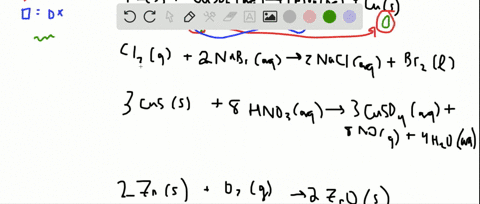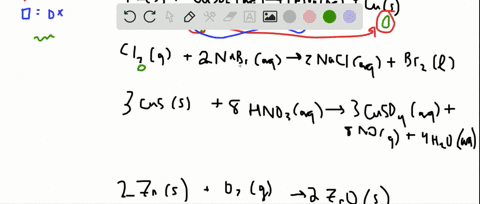
Solved For Each Of The Following Balanced Oxidation Reduction Reactions I Identify The Oxidation Numbers For All The Elements In The Reactants And Products And Ii State The Total Number Of Electrons Transferred In
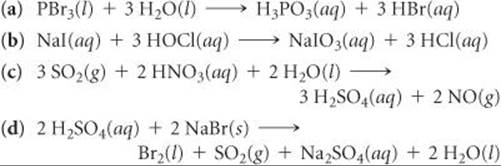
Exercises Electro Chemistry Chemistry The Central Science

Solved For Each Of The Following Balanced Oxidation Reduction Reactions I Identify The Oxidation Numbers For All The Elements In The Reactants And Products And Ii State The Total Number Of Electrons Transferred In
Solved Problem 20 18 Part A Indicate Whether The Following Chegg Com
Solved Write The Balanced Chemical Equation For The Redox Reaction That Would Take Place In This Voltaic Cell 2agt Mg 2ag Mg2 Calculate The E Celi For This Battery Would Production
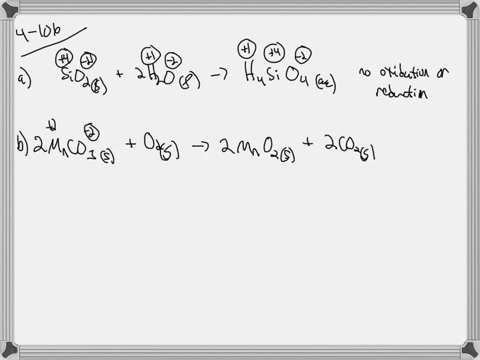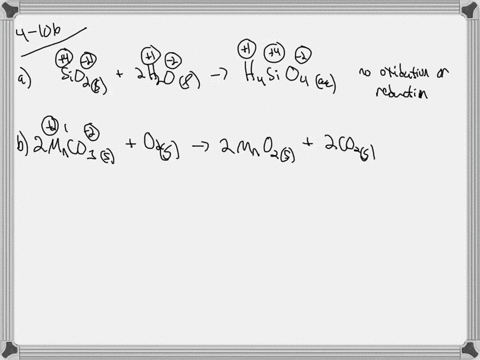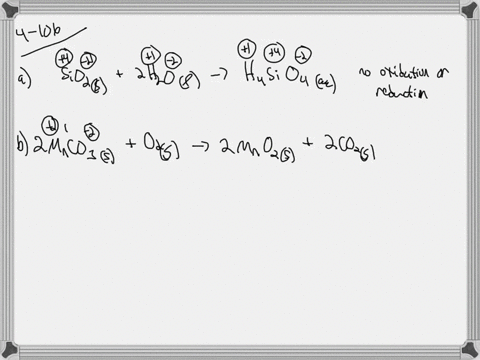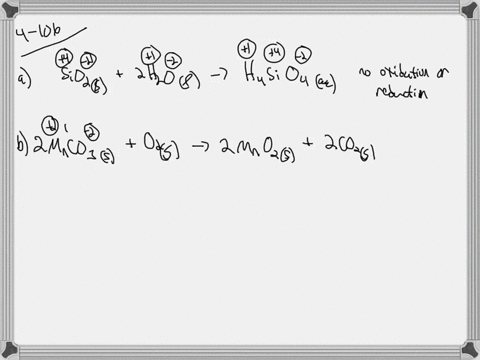
Solved For Each Of The Following Balanced Oxidation Reduction Reactions I Identify The Oxidation Numbers For All The Elements In The Reactants And Products And Ii State The Total Number Of Electrons Transferred In
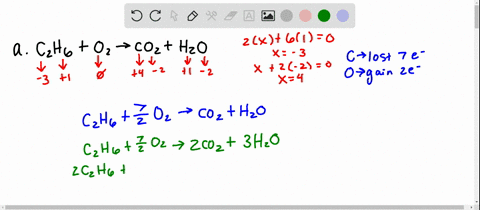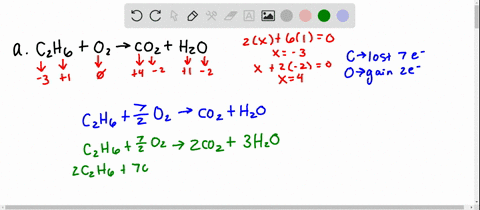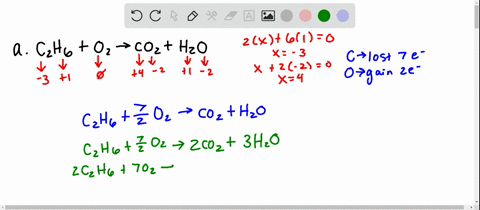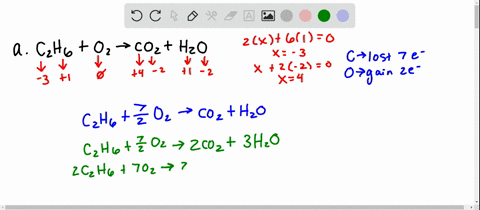
Solved For Each Of The Following Balanced Oxidation Reduction Reactions I Identify The Oxidation Numbers For All The Elements In The Reactants And Products And Ii State The Total Number Of Electrons Transferred In
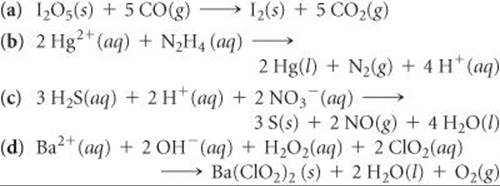
Exercises Electro Chemistry Chemistry The Central Science
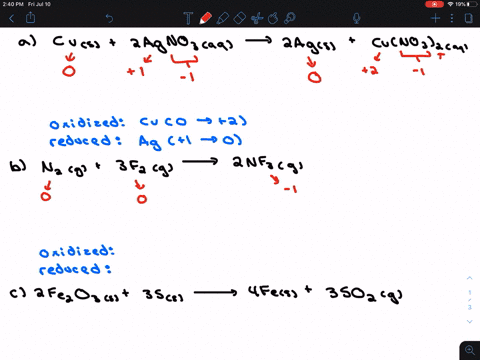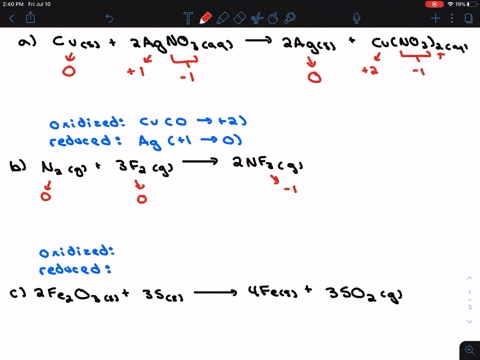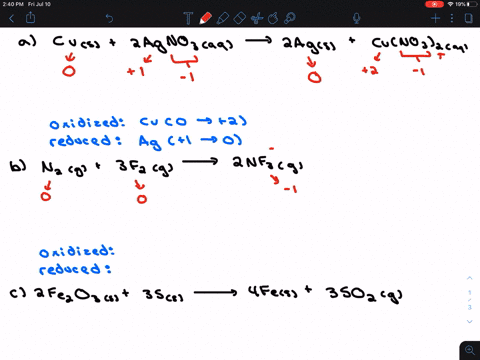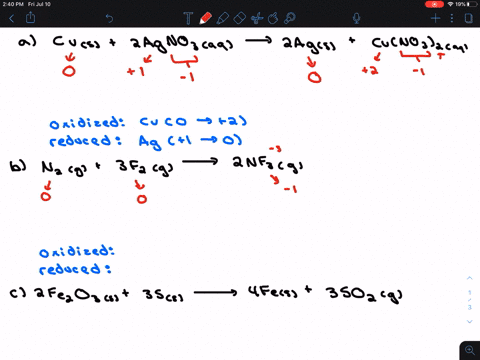
Solved For Each Of The Following Balanced Oxidation Reduction Reactions I Identify The Oxidation Numbers For All The Elements In The Reactants And Products And Ii State The Total Number Of Electrons Transferred In
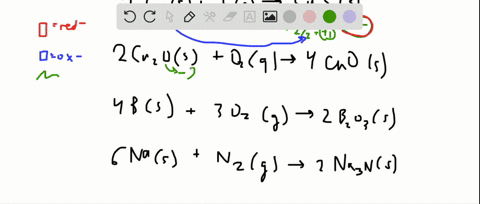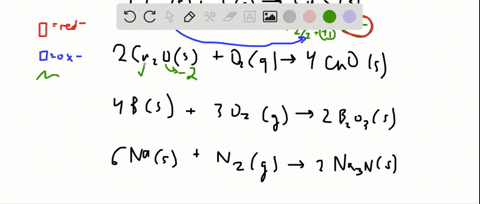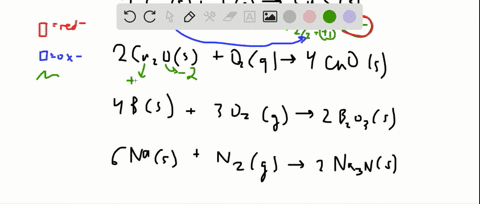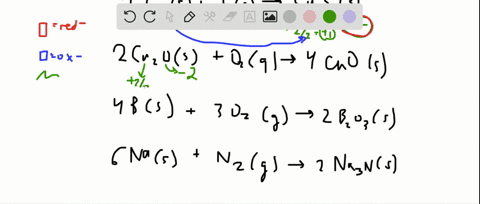
Chapter 18 Oxidation Reduction Reactions And Electrochemistry Video Solutions Introductory Chemistry Numerade
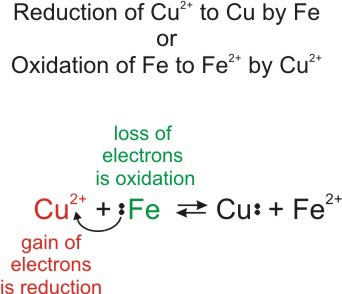
Chapter 11 Electron Transfer Reactions And Electrochemistry
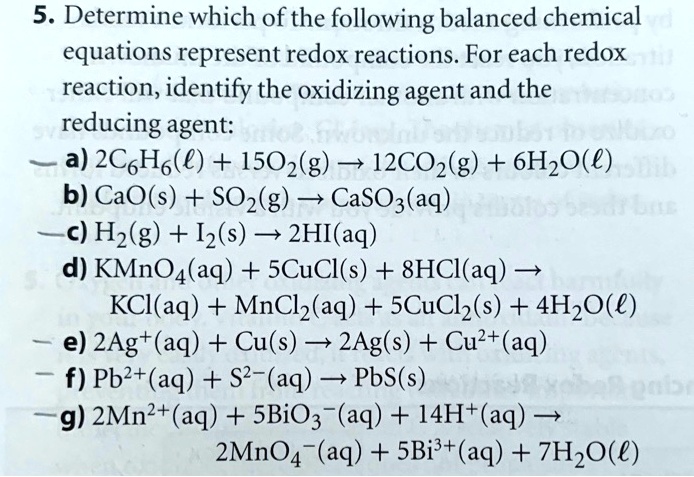
Solved 5 Determine Which Of The Following Balanced Chemical Equations Represent Redox Reactions For Each Redox Reaction Identify The Oxidizing Agent And The Reducing Agent A 2c6h6 A 1502 G 12co2 G 6hzo E
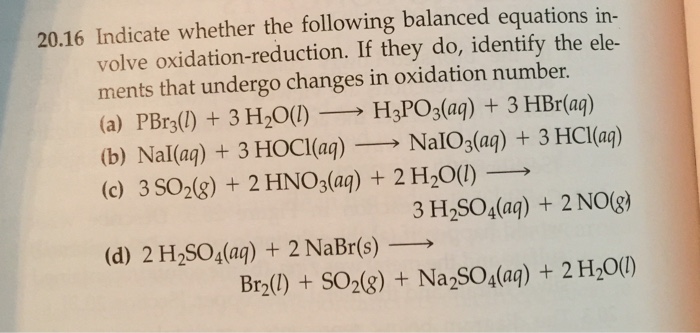
Solved Indicate Whether The Following Balanced Equations Chegg Com
Solved Indicate Whether The Following Balanced Equations Chegg Com

Writing Half Reactions Of Redox Reactions Chemistry Study Com

Solved For Each Of The Following Balanced Oxidation Reduction Reactions I Identify The Oxidation Numbers For All The Elements In The Reactants And Products And Ii State The Total Number Of Electrons Transferred In
Comments
Post a Comment