Deviations to the Electron Affinity Trend Occur Because of:
For a particular energy level removing an electron from the s subshell would require a higher first ionization energy. Since a half-filled p subshell is more stable carbon has a greater affinity for an electron than nitrogen.

3 4 Trends In Electron Affinity And Metallic Character Chemistry Libretexts
Electron configuration and orbital diagrams are helpful tools to help determine the primary effects involved in the trends as well as deviations from the trend.

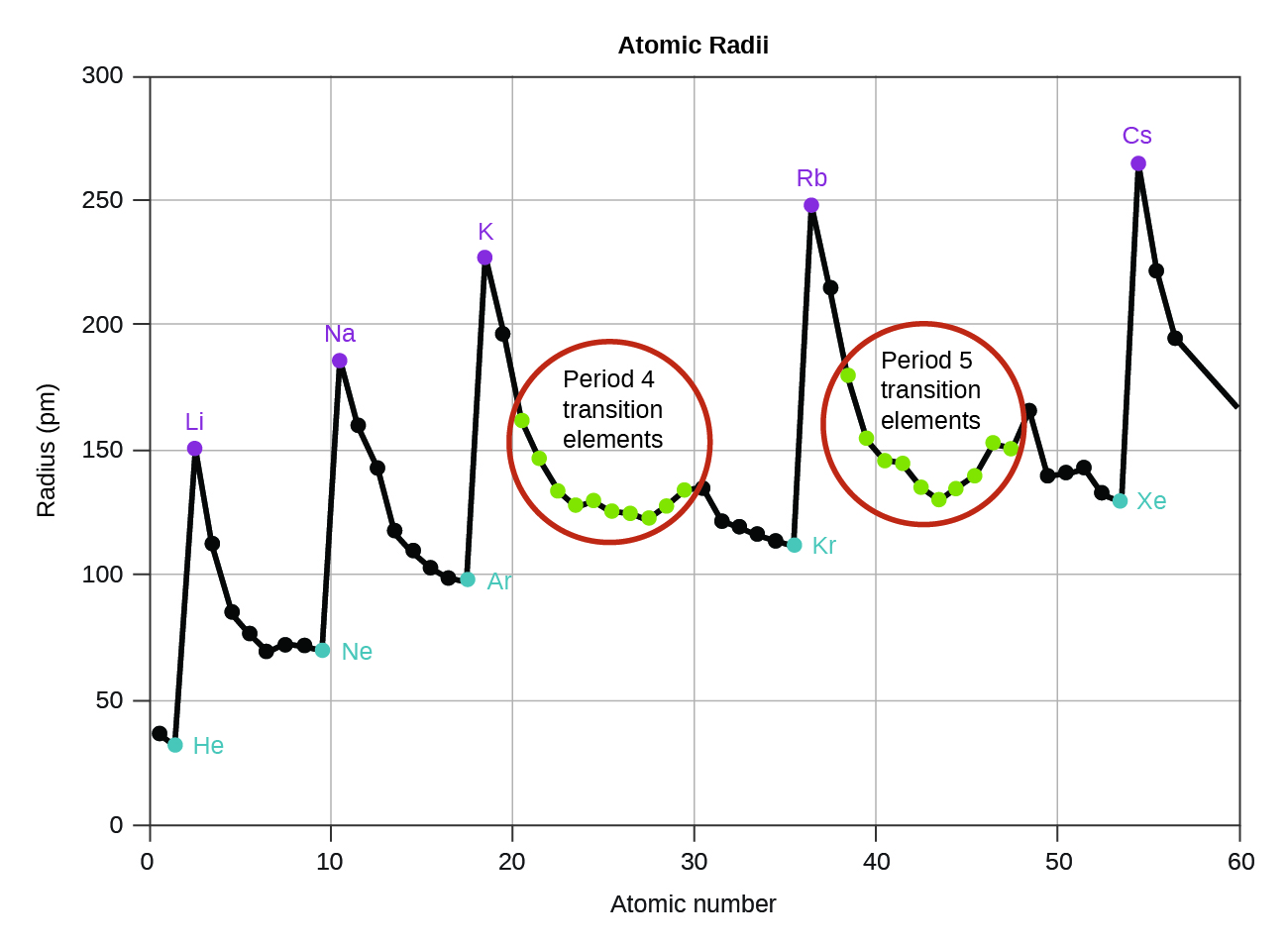
. Minor deviations from this trend can be explained in terms of particularly stable electronic configurations called pseudo noble gas configurations in either the parent atom or the resulting ion. Most exceptions to the trend of decreasing radius moving to the right within a period occur in the Select the correct answer below. For beryllium the first ionization potential electron comes from the 2s orbital although ionization of boron involves a 2p electron.
The overall trend across a period occurs because of increased nuclear attraction. Electron affinity the energy associated with forming an anion is more favorable exothermic when electrons are placed into lower energy orbitals closer to the nucleus. S block p block d block There are no exceptions to this trend.
However deviations from the trends may occur in a variety of properties. This similarity occurs because the members of a group have the same number and distribution of electrons in their valence shells. In other words when the electron is added to a neutral atom the energy is either released or absorbed.
Electron affinity the energy associated with forming an anion is more favorable exothermic when electrons are placed into lower energy orbitals closer to the nucleus. Electron affinity the energy associated with forming an anion is more favorable exothermic when electrons are placed into lower energy orbitals closer to the nucleus. Adding an electron to an element with 3 electrons in p orbitals means the new electron must form a pair in one of the subshells a high-energy proposition.
Tramwayniceix and 4 more users found this answer helpful. A general trend in electron affinity is that as one goes from. In which of the following element blocks does the atomic radius generally not tend to increase when moving down within a group.
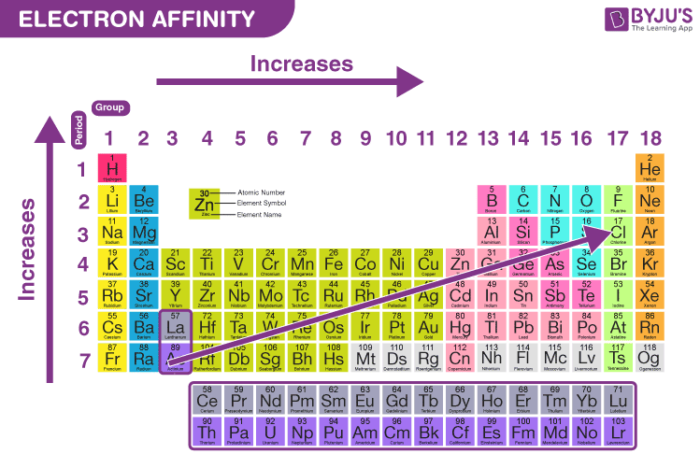
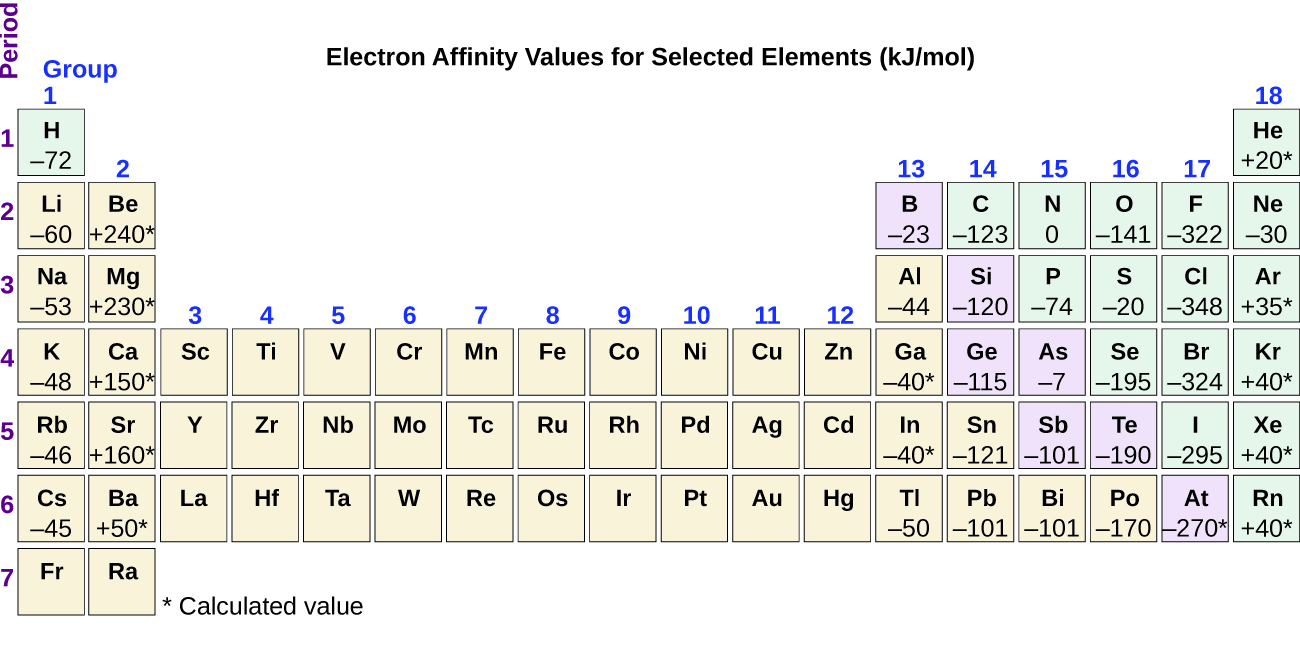
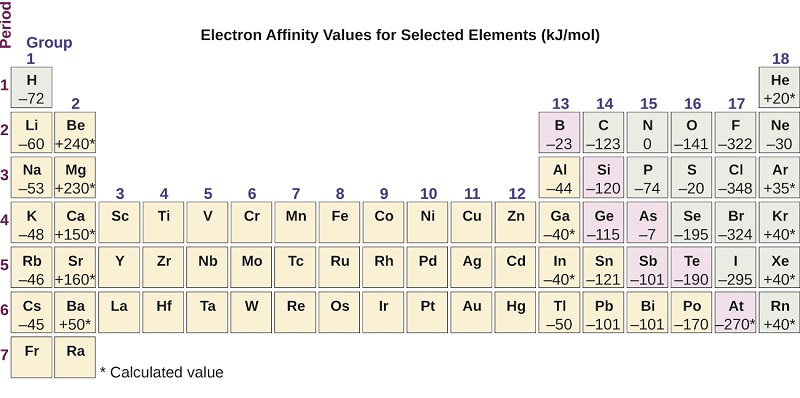
The reaction that occurs when an atom takes an electron may be represented as. The reason for the discrepancy is due to the electron configuration of these elements and Hunds rule. As seen in the table of electron affinities below the electron affinity of most elements is exothermic but the electron affinities for all of the noble gases are endothermic.
Electron affinity decreases or increases across a period depending on electronic configuration. The elements in groups vertical columns of the periodic table exhibit similar chemical behavior. Therefore electron affinity becomes increasingly negative as we move left to right across the periodic table and decreases as we move down a group.
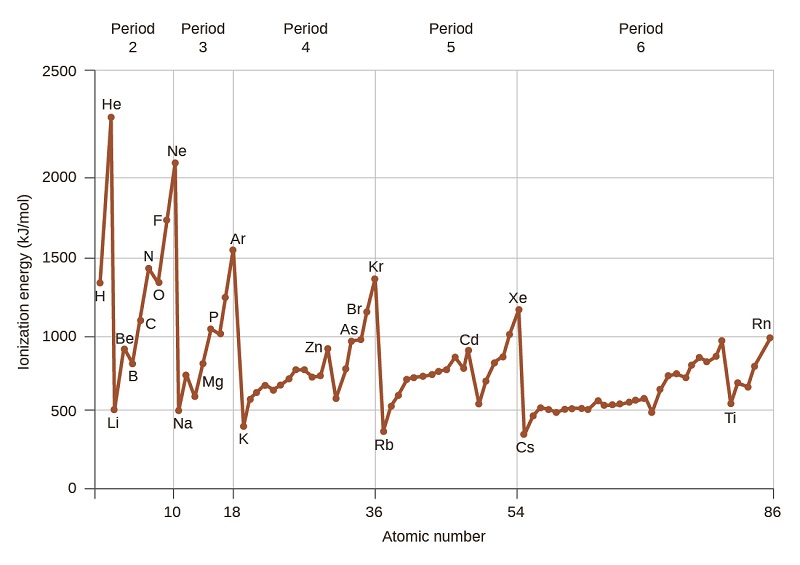
And this amount of energy change ΔE is called electron affinity. Electrons are added increasingly farther from the nucleus so the attractive force between the nucleus and electrons decreases. Deviations to the trends in first ionization energy occur because of which of orbital symmetry.
The electron affinity for an element with a full s shell is low since the new electron would need to be in the higher-energy p orbital. Orbital symmetry For example oxygen has a slightly lower ionization energy than nitrogen because nitrogen would lose half-full p orbitals while oxygen would gain half-full p orbitals. Deviations to the trends in first ionization energy occur because of which of the following.
The overall trend across a period occurs because of increased nuclear attraction. The Electronegativity Trend As a practical example of electronegativity in action consider the fact that an atom of chlorine has a higher electronegativity value than an atom of hydrogen. 87 Periodic Trends and Variation of Properties.
It is the energy change that occurs when an electron is added to a gaseous atom. This makes it different from electron affinity because electron affinity refers to the actual energy released when atoms end up gaining an electron. These variations can usually be explained by electron-electron repulsion nucleus-electron attraction or shielding.
Therefore electron affinity becomes increasingly negative as we move left to right across the periodic table and decreases as we move down a group. Electron affinity reflects the ability of an atom to accept an electron. However there are also other patterns in chemical properties on.
The electron affinity trend describes the trend across the periodic table and describes how much energy in an atom is released or spent when an electron is added to a neutral atom or the energy change that occurs when an electron is added to a neutral atom. For both nitrogen and oxygen the electron comes from the 2p orbital but the spin is the same for all 2p nitrogen electrons while. Electron affinities are difficult to measure.
In general elements with the most negative. Therefore electron affinity becomes increasingly negative as we move left to right across the Periodic Table and becomes less negative as we move down a group. This occurs because of the same subshell rule that governs ionization energies.
It becomes more negative Deviations to the electron affinity trend occur because of. Electron affinity the energy associated with forming an anion is more favorable exothermic when electrons are placed into lower energy orbitals closer to the nucleus. X e X energy.
The stability of the atom s electron configuration If the electron affinity for an element is negative this means that energy is released when an atom gains an electron. Obviously the halogens which are one electron away from a noble gas electron configuration. The electron affinity trend describes how as one follows the periodic table.
Electron affinity is the amount of energy change ΔE that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The amount of energy released when an electron is added to a neutral atom to form an anion is called electron affinity. Energy required to add an electron to a gaseous atom to form an anion.
Atoms with stronger effective nuclear charge have greater electron affinity. The electron affinity EA of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. Therefore electron affinity becomes increasingly negative as we move left to right across the Periodic Table and becomes less negative as we move down a group.
Electron affinity increases going left to right across a period. The value of electron affinity usually becomes less negative on descending a group of the periodic table. Daniel NelsonPRO INVESTOR.

3 4 Trends In Electron Affinity And Metallic Character Chemistry Libretexts

Periodic Trends And Coulomb S Law Video Khan Academy
What Is Electron Affinity Definition Trends Equation With Videos

Ch 121 Chapter 3 Part 2 3 4 3 5 3 6 Flashcards Quizlet
What Are The Exceptions In Trends Of Electron Affinity In The Periodic Table As We Move Along The Period Quora
8 7 Periodic Trends And Variation Of Properties General Chemistry For Gee Gees

Periodic Trends Boundless Chemistry

Periodic Trends Boundless Chemistry
8 7 Periodic Trends And Variation Of Properties General Chemistry For Gee Gees

What Is Electron Affinity Definition Trends Equation With Videos
In The Periodic Table Why Does Group 3a Have Less Ionization Energy Then Group 2a Quora

Question Video Determining The Order That Rusting Will Occur In An Experiment Nagwa
Why Does Electronegativity Increase From Left To Right Across The Periodic Table Quora

7 4 Ionization Energy Chemistry Libretexts

8 4 Electron Configurations Valence Electrons And The Periodic Table Chemistry Libretexts
3 4 Trends In Electron Affinity And Metallic Character Chemistry Libretexts
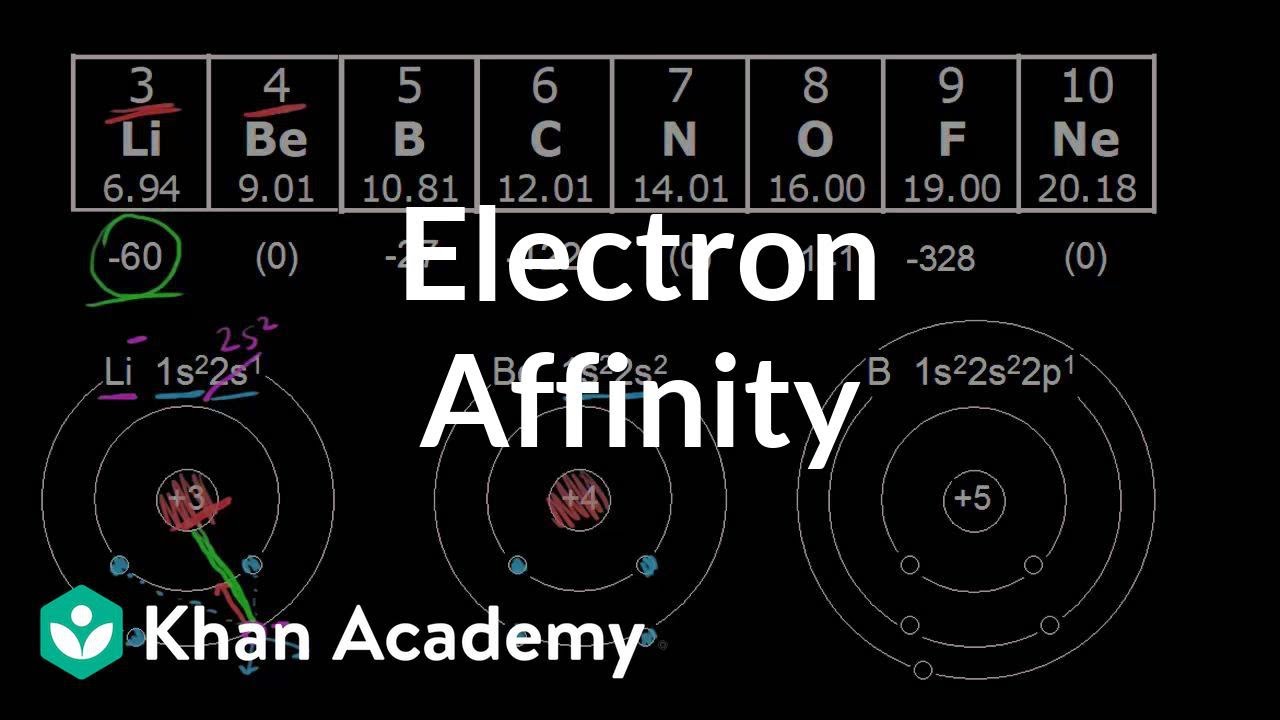
Electron Affinity Period Trend Video Khan Academy

Comments
Post a Comment